Schreiner MediPharm Syringe Label Meets EU Safety Standards
- Published: May 22, 2013
BLAUVELT, NY | Schreiner MediPharm, a provider of specialty pharmaceutical labeling solutions, announces Needle-Trap, a system designed to protect healthcare providers from accidental needlestick injuries from disposable syringes. This product is expected to help US pharma manufacturers doing business in Europe comply with a new EU directive on the prevention of sharps (syringe) injuries in the hospital and healthcare sector. 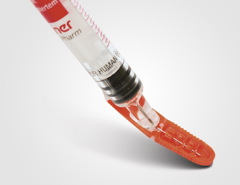
The system, which meets the upgraded safety mandates of the new EU Council Directive and also has been awarded FDA 510(k) clearance for marketing in the US, features a safety mechanism integrated as a component of the self-adhesive syringe label. Said to be compact and economical, design allows the blood-contaminated needle to be secured simply and safely following its use. It also can be operated easily by healthcare personnel in a controlled, single-handed fashion, company says. After the needle has locked into the plastic trap—an action clearly perceptible by a clicking sound—it is irreversibly protected.
Reportedly, system can be easily integrated into conventional packaging and labeling systems, since it is processed and dispensed like a single-layer label, and it is adaptable to all standard syringe dimensions. It requires no additional storage space and is housed in common secondary packaging. To reduce the risk of medication errors, optional detachable documentation labels also are available.
This email address is being protected from spambots. You need JavaScript enabled to view it.